How to Find Moles
For example if you have 170 mol of NaCl then 170 mol 58443 g 1 mol 994 g It has been found that 1 mol of any gas at STP. It can be used to measure the products obtained from the chemical reaction.
Converting Between Moles Atoms And Molecules Teaching Chemistry Chemistry Textbook High School Chemistry
In general if you know the mass of a substance and the number of mole you can calculate the molecular weight MWt.

. Molar mass O2 162 32gmol. To go from moles to grams multiply by the formula mass. To get the result of your problem according to the above steps you can use mole calculator.
First calculate the number of moles of NaCl. As you can see calculations for molality are straightforward. For hydrogen chloride the molar mass is 1007 35453 36460 gmol.
Molarity moles of solute litres of solution First of all before you can use this equation you need to know how many moles of solute are there in the solution. Find the molarity by calculating the number of moles of the solute. For finding out this you have.
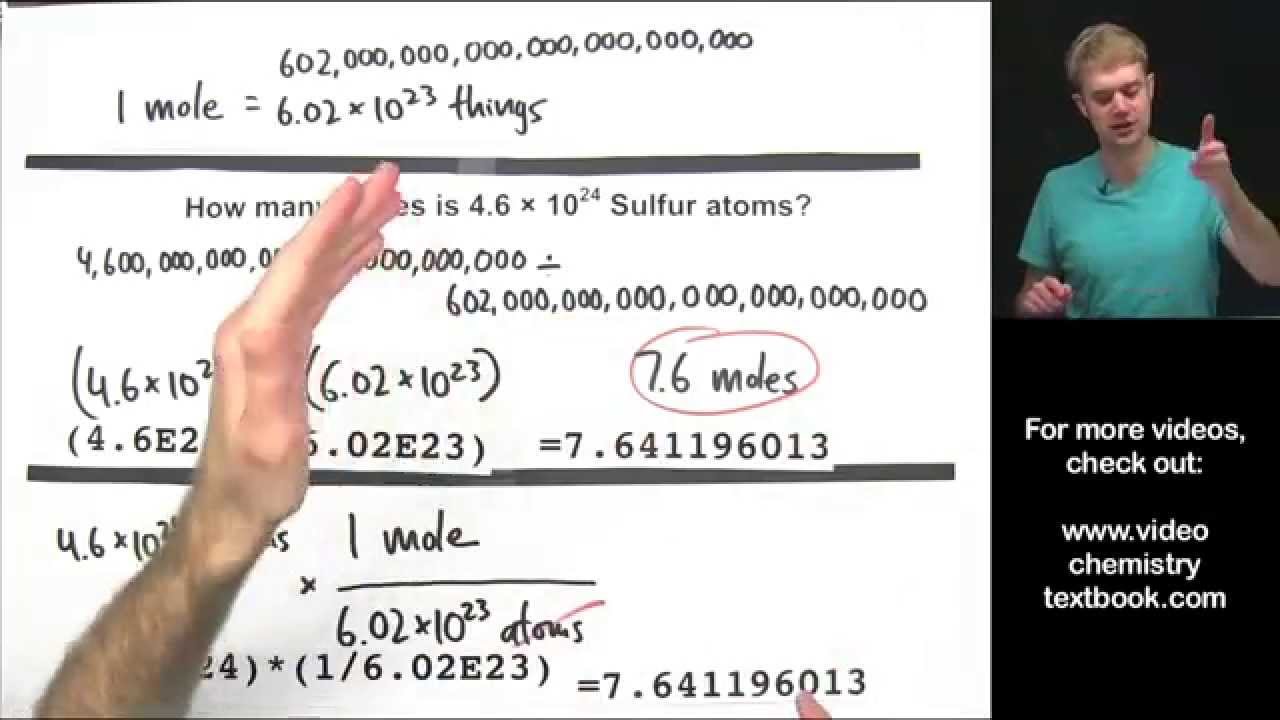
By using a special number called Avogadros Constant 6022e23 you can find out the Molar Mass of a single. Convert the moles of substance to desired units of measure. The unit is denoted by mol.
The result is the number of moles in your element or compound. M n M 102. Use 4 tablespoons of this concoction in a gallon of water and soak the tunnels and the entrances.
M The molar mass. Solved Example on the Number of Moles of a Substance. Mix up a spray of 3 parts castor oil to 1 part dish detergent.
1Calculate mass of O2 having moles equal to 102 mol. The general formula for this is written as. One mole of any substance is equal to the value of 6023 x 1023 Avagadro number.
This video explains how to calculate the number of moles of an element given the mass as well as how to calculate the mass given the number of moles. Remember to find the number of. Number of moles mass relative formula mass This can be rearranged to find.
Mole mass of a substance or material molar mass. In this animated lecture I will teach you about the 3 different easy methods to calculat. 3646 grams is the mass of one mole of hydrogen chloride.
In order to find the molarity you need to divide 009 mol the number of moles of the solute NaCl by 08 L the volume of the solution in liters. The Avogadro number is. Molarity moles of solute liters of solution 009.
One mole of ibuprofen C 13 H 18 O 2 has a mass. How to find mass from moles and molar mass examples. The molality of the NaCl solution is 025 molal.
Mole Concept- A mole is defined as the amount of a substance that contains exactly the Avogadro number of elementary entities of the given substance. Moles are extremely useful for finding the amount of atoms in a substance. M a s s m o l e s M W t Rearrange that equation algebraically to get.
A mole of a substance or a mole of particles is defined as exactly 60221407610²³ particles which may be atoms molecules ions or electrons. One mole of NH 3 molecules which has a relative molecular mass Mr of 17 has a mass of 17 g and half a mole has a mass of 85 g. The mole is important because it allows.
Mole calculations This equation shows how relative formula mass number of moles and mass are related. How many moles are in 250 grams of water. For glucose the molar mass is 720642 12084.
N The number of moles m The given mass and. Dip an ear of corn in roofing tar. N 102 mol.
This lecture is about how to find the number of moles in chemistry.

Molarity M The Concentration Of A Solution As The Number Of Moles Of Solute Chemistry Education Biology Notes Chemistry

Mole Ratio Easy Science 10th Grade Science Chemistry Notes Easy Science

Partial Pressures Of Gases And Mole Fractions Chemistry Tutorial Mole Fraction Chemistry Fractions

No comments for "How to Find Moles"
Post a Comment